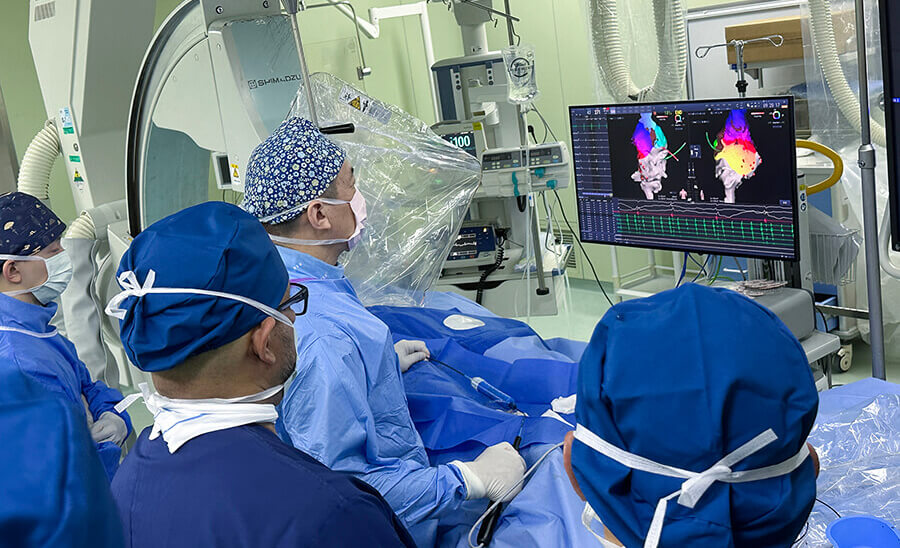
We are pleased to announce that APT Medical’s series of pioneering electrophysiology (EP) pulse field ablation (PFA) products has been officially approved by the National Medical Products Administration (NMPA). This portfolio includes:
• AForcePlus™ Contact Force Sensing PFA Ablation Catheter
• Pulstamper™ Circular PFA Ablation Catheter
• Pulse Field Ablation Generator
• HT Viewer® 3D Mapping System (Pro version)
This approval marks a significant milestone for APT Medical, positioning us as the world’s first company to offer an integrated all-3D pulse field ablation solution. Combining advanced electromagnetic catheters, a cutting-edge ablation system, and a comprehensive 3D mapping platform. These innovations enable precise real-time catheter navigation, contact force feedback during ablation, optimized ablation strategies, and most importantly, the adoption of PFA energy, ensuring safe, efficient, and minimally invasive atrial fibrillation (AF) procedures with conscious sedation.
Clinical trials of our PFA system have demonstrated an 83.39% 12-month AF ablation success rate (defined as freedom from atrial fibrillation, atrial flutter, or atrial tachycardia without the use of Class I, III, or IV antiarrhythmic drugs during follow-up) and a 0% major adverse event rate. These results underscore the exceptional safety and efficacy of our solution for paroxysmal AF patients.
With these advancements, APT Medical sets a new standard in AF treatment, delivering cutting-edge technology that ensures better patient outcomes with unparalleled safety, efficacy, and efficiency.